Products
Built multiple sets of modular, multi-functional intelligent drug automatic synthesis platform
18F-DPA-714 material
Keywords: synthetic equipment disposable consumables
Classification:
Tracers

Hotline:
18F-DPA-714 material
Graphic Details
18F-DPA-714 Materials
1. Drug Name (Generic Name, Chemical Name, English Name, Pinyin, and rationale for custom names if any)
Generic Name: 18F-DPA-714
Chemical Name: N-[2-[(4 - chlorophenyl ) methyl ]-6 - methylpyridine - 3 - yl ]-2-(18F) fluoroacetamide
English Name: 18F-DPA-714 (Full English Name: N-[2-[(4-chlorophenyl) methyl]-6-methylpyridin-3-yl]-2-(18F) fluoroacetamide )
Pinyin: yī bā F-DPA-qī yī sì 。
2. Drug Chemical Structure, Molecular Weight, Molecular Formula
Chemical Structure:
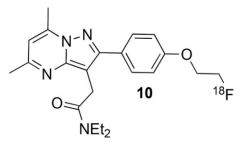
Molecular Weight: 294.72
Molecular Formula: C 15 H 12 ClFN 2 O
Rationale
Neuroinflammation plays a key role in the occurrence and development of numerous neurological diseases, such as Alzheimer's disease, Parkinson's disease, epilepsy, and autoimmune encephalitis. Microglia, as the immune cells of the central nervous system, are important markers of neuroinflammation when activated. 18F - DPA - 714 As a specific microglia imaging agent, it can bind with high affinity to the 18kD translocator protein ( TSPO ) . Through positron emission tomography ( PET ) technology, 18F - DPA - 714 it can non-invasively and real-time monitor the activation state of microglia in vivo, thus providing important imaging evidence for the early diagnosis, disease assessment, and treatment effect monitoring of neurological diseases.
Domestic Research and Development Status
The project "Experimental Study of Microglia Molecular Imaging of 18F - DPA714 in the Inflammatory Mechanism of AD and the Monitoring of Anti-inflammatory Intervention Efficacy" undertaken by Professor Jun Zhao's team at Fudan University successfully synthesized the 18F - DPA - 714 neuroinflammation molecular probe. The optimized labeling conditions are that 6.7 mg/ml precursor solution and K - F - K2.2.2 mixture react at 105 ℃ for 10 min, with a radiochemical yield of 40.3 ± 5.1%, radiochemical purity ≥99%, and radiochemical synthesis time of about 20 minutes.
Domestic Application Status
- Diagnosis of autoimmune encephalitis: In March 2024, the Department of Nuclear Medicine and the Department of Neurology of Ruijin Hospital collaborated to publish a paper in the journal Radiology, which found that in 25 confirmed patients with autoimmune encephalitis (AIE), the positive detection rate of PET was 72%, while that of MRI was only 44%, suggesting that 18F - DPA - 714 PET has potential added value in detecting AIE.
- Preoperative localization of intractable epilepsy: In a research paper published in the internationally renowned journal CNS Neuroscience & Therapeutics, Professor Jie Lu's team retrospectively included 43 patients with intractable epilepsy who underwent surgery and analyzed their preoperative 18F - FDG and 18F - DPA - 714 dual tracer PET/MRI imaging data, finding that 18F - DPA - 714 is expected to become an important tool for preoperative localization of epileptogenic zones and precise definition of lesions in patients with intractable epilepsy.
- Parkinson's disease research: The Department of Nuclear Medicine of Harbin Medical University No. 1 Hospital successfully synthesized the 18F - DPA - 714 neuroinflammation imaging agent and applied it to the research of Parkinson's disease, promoting the application of neuro-nuclear medicine molecular imaging in the province.
International Application Status
18F - DPA - 714 is also widely used in the research of neurological diseases abroad. For example, in the study of Alzheimer's disease, 18F - DPA - 714 PET imaging showed that there was a significant increase in the uptake of 18F - DPA - 714 in brain areas related to neuroinflammation in patients, and the degree of uptake was related to the severity of the disease. In the study of "long COVID" patients, 18F - DPA - 714 PET was used to observe the levels in the patients' brains to assess microglial activation inflammation in the brain nerves, and it was found that microglia were highly active in the brains of "long COVID" patients. In addition, in the research of other neurological diseases such as multiple sclerosis and stroke, 18F - DPA - 714 has also been used to observe changes in neuroinflammation, providing important imaging information for the study of the pathophysiological mechanisms and therapeutic interventions of the diseases.
4. Drug Instructions
Generic Name: 18F-DPA-714
Chemical Name: N-[2-[(4 - chlorophenyl) methyl]-6 - methylpyridine - 3 - yl]-2-(18F) fluoroacetamide
English Name: 18F-DPA-714 (Full English Name: N-[2-[(4-chlorophenyl) methyl]-6-methylpyridin-3-yl]-2-(18F) fluoroacetamide )
Pinyin: yī bā F-DPA-qī yī sì
【Composition】
The main components and their chemical names are: TSPO protein and its structural formula
【Properties】
This product is a colorless and clear liquid
【Indications】
18F-DPA-714 is used for epilepsy, neuroinflammation, metabolic diseases, brain tumors and degenerative diseases, etc.
【18F-DPA-714 PET/CT Brain Imaging Procedure】
I. Pre-examination preparation
Patient preparation
Fasting for 6 hours before the examination, maintaining blood glucose ≤7.0 mmol/L (insulin injection is required for adjustment if necessary)
Suspend drugs that may interfere with TSPO expression (such as glucocorticoids)
Drug preparation and injection
Calculate the dose of 18F-DPA-714 according to body weight (adults usually use 185-370 MBq), and inject intravenously slowly.
II. Tracer injection and resting period
Rest after injection
Rest for 60 minutes, avoid activities, strong light or external stimuli, to ensure that the tracer and TSPO are fully combined.
Maintain a quiet environment to reduce non-specific uptake in the brain.
PET/CT acquisition
Mode: Three-dimensional dynamic or static acquisition (select according to research needs).
Time: Static scanning takes about 10-15 minutes, dynamic scanning needs to be extended to more than 60 minutes.
Range: Full brain coverage, key areas (such as the temporal lobe, hippocampus, etc.) need to ensure clear signals.
Scanning should be performed on time 50 minutes after injection of the tracer. CT and PET acquisition parameters and reconstruction methods should be consistent with brain 18F-FDG imaging. The scanning group needs to strictly control the injection time and scanning time, and strictly record the scanning time.
Image interpretation and analysis: Interpret images promptly.
Report: Issue a report promptly.
Follow-up arrangements: 18F-DPA-714 and 18F-FDG brain imaging should be spaced at least 10 half-lives (or 20 hours).
This product can only be used in medical institutions with a "Radioactive Drug Use License".
【Adverse reactions】
None found yet.
【Contraindications】
None found yet.
【Precautions】
If discoloration or turbidity occurs, stop using this product.
This product can only be used in medical institutions with a "Radioactive Drug Use License".
【Use in pregnant and lactating women】
Contraindicated in pregnant and lactating women.
【Pediatric use】
Reduce the dose appropriately according to body weight.
【Specifications】
0.37~7.40GBq.
【Storage and packaging】
This product is sealed in a 30ml vial and placed in a lead container.
【Shelf life】
6 hours from the calibration time.
【Manufacturer】
Name: Hangzhou Jirui Technology Co., Ltd.
Address: No. 319, Shenjia Road, Gongshu District, Hangzhou, Fengqigu Yunzhang Industrial Park
Post code: 234122
Telephone number: 0571-87701916
Previous Page
18F-AV1451 material
Next Page
Related Products
Consulting