Products
Built multiple sets of modular, multi-functional intelligent drug automatic synthesis platform
18F-JR1002 material
Keywords: synthetic equipment disposable consumables
Classification:
Tracers

Hotline:
18F-JR1002 material
Graphic Details
18F-JR1002 Materials
1. Drug Name (Generic Name, Chemical Name, English Name, Pinyin, and rationale for custom names if any)
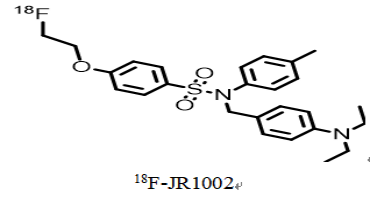
Generic Name: 18F-JR1002
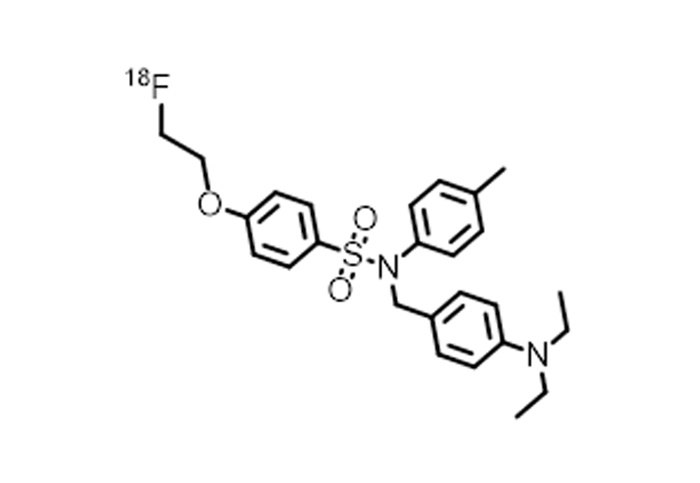
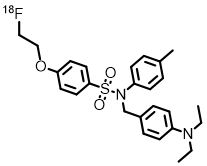
Chemical Name: N-[4-( diethylamino ) benzyl ]-4-[2-( fluoro -18F) ethoxy ]-N-( p-tolyl ) benzenesulfonamide
English Name: N-(4-(diethylamino)benzyl)-4-(2-(fluoro- 18 F)ethoxy)-N-(p-tolyl)benzenesulfonamide
Pinyin: 18F-JR1002
2. Drug chemical structure, molecular weight, molecular formula
Chemical Structure:
3. Rationale ( Literature on the research and application of this product at home and abroad )
Cannabinoid type 2 receptor (CB2R) belongs to the G protein-coupled receptor (GPCRs) family. Together with cannabinoid type 1 receptor (CB1R), it constitutes the endocannabinoid system (ECS) through its respective signaling pathways, which is a complex and pleiotropic endogenous signaling system. Since the cloning of CB2R in 1993, CBR has been largely distinguished based on its biodistribution. CB1R is mainly highly expressed in the nervous system and has neuromodulatory properties, while CB2R is widely distributed in the peripheral immune system and has immunomodulatory properties.
Positron emission tomography (PET) is a non-invasive and highly sensitive molecular imaging technique that allows in vivo quantification of biochemical and pharmacological processes in health and disease. By using radioactive PET tracers, in vivo imaging can be achieved at the molecular level to obtain quantitative information on target engagement and receptor occupancy at a given drug dose. PET imaging combined with specific probes can provide real-time information on tracer accumulation in the region of interest, playing a crucial role in precision medicine and quantitative pharmacokinetic studies. Therefore, PET molecular imaging targeting CB2R receptors is expected to have the potential in personalized diagnosis and treatment monitoring of various diseases such as neuroinflammation, neurodegeneration, and tumors.
4 Research methods, experimental conditions, etc., of imaging or simulated clinical functional determination tests of target organs and whole-body imaging in experimental animals, and imaging or functional determination results at various time phases of observation
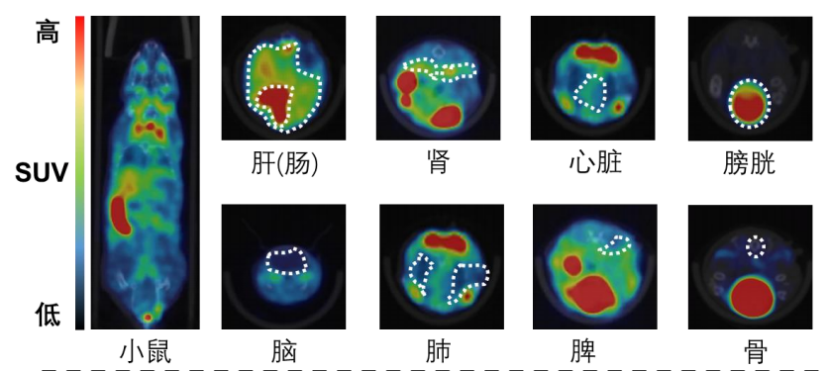
I. Whole-body imaging and delayed imaging of experimental animals
1 Materials and Methods
1.1 Experimental animals
The experimental animals were 2 mice, weighing about 25g, male, obtained from the Zhejiang University laboratory. Images were collected at multiple time points after drug injection to obtain the drug distribution map in vivo. After imaging, the experimental animals gradually woke up and recovered to normal, with good diet, defecation, urination, and mental state.
The following figure shows 30min imaging images
II. Acute toxicity test
Ten healthy mice (6-8 weeks old, weighing 20-25g), with equal numbers of males and females, were selected. 0.5mL of 18F-JR1002 with an activity of 1mCi was injected into the tail vein. The control group consisted of 10 healthy mice (6-8 weeks old, weighing 20-25g), with equal numbers of males and females, and 0.5mL of saline was injected into the tail vein. The survival status of the two groups of mice was observed. There was no statistically significant difference in the survival status of the two groups of mice within 7 days.
5. Drug Instructions
Generic Name: 18F-JR1002
Chemical Name: N-[4-(diethylamino)benzyl]-4-[2-(fluoro-18F)ethoxy]-N-(p-tolyl)benzenesulfonamide
English Name: N-(4-(diethylamino)benzyl)-4-(2-(fluoro-18F)ethoxy)-N-(p-tolyl)benzenesulfonamide
Pinyin: shíbā F-JR yī líng líng èr
【Ingredients】
The main components and chemical names of this product are: G protein and its structural formula
【Properties】
This product is a colorless and clear or slightly yellow and clear solution.
【Indications】
18F-JR1002 is used for tumor diagnosis and staging assessment, cardiovascular disease imaging, and monitoring of bone lesions or bone metastases.
【18F-JR1002 PET/CT Brain Imaging Procedure】
Pre-examination preparation
Fasting requirements: Subjects need to fast for 4-6 hours (water is allowed) to avoid blood glucose fluctuations interfering with the distribution of the imaging agent.
Blood glucose monitoring: Blood glucose needs to be checked before injection, usually requiring control to ≤11mmol/L.
Contraindicated adjustments: Suspend drugs that may interfere with the results (such as insulin, hormone drugs), and avoid strenuous exercise.
Imaging agent injection and resting period
Intravenous injection: The 18F-JR1002 tracer is administered intravenously, and the dose is adjusted according to body weight and examination purpose.
Resting period: After injection, rest in a quiet, dark environment for 45-60 minutes, avoiding activity or mental stimulation during this period to ensure the specific distribution of the tracer in brain tissue.
Brain Imaging Scan
Positioning: The patient lies supine on the scanning bed, with the head fixed using a dedicated headrest, maintaining steady breathing and stillness.
CT Scan: A low-dose CT scan (approximately 10 minutes) is performed first to obtain anatomical images of the brain.
PET Scan: This is followed by a PET scan (approximately 20-30 minutes) to detect the metabolic distribution and targeted binding of 18F-JR1002.
Image Processing and Diagnosis
Data Fusion: The PET functional images and CT anatomical images are fused using software to generate a three-dimensional brain metabolic image.
Clinical Interpretation: A nuclear medicine physician, considering metabolic activity, lesion location, and patient history, will issue a diagnostic report.
Post-Examination Precautions
Radiation Protection: It is recommended to drink plenty of water and urinate frequently after the examination to accelerate the excretion of the radioactive tracer.
Result Acquisition: The formal report is usually available within 1-2 working days.
Follow-up Arrangements: Brain imaging with 18F-JR1002 and 18F-FDG should be spaced at least 10 half-lives (or 20 hours) apart.
This product can only be used in medical institutions with a "Radioactive Pharmaceutical Use License".
[Adverse Reactions]
None have been found.
[Contraindications]
None have been found.
[Precautions]
If discoloration or turbidity occurs, discontinue use.
This product can only be used in medical institutions with a "Radioactive Pharmaceutical Use License".
[Use in Pregnant and Lactating Women]
Contraindicated in pregnant and lactating women.
[Pediatric Use]
Reduce the dose appropriately according to body weight.
[Specifications]
0.37~7.40GBq.
[Storage and Packaging]
This product is sealed in a 30ml vial and placed in a lead container.
[Expiration Date]
6 hours from the calibration time.
[Manufacturer]
Name: Hangzhou Jirei Technology Co., Ltd.
Address: No. 319, Shenjia Road, Gongshu District, Hangzhou, Fengqi Valley Yunzhang Industrial Park
Postal Code: 234122
Telephone Number: 0571-87701916
Previous Page
18F-FPABZA material
Next Page
Related Products
Consulting