Products
Built multiple sets of modular, multi-functional intelligent drug automatic synthesis platform
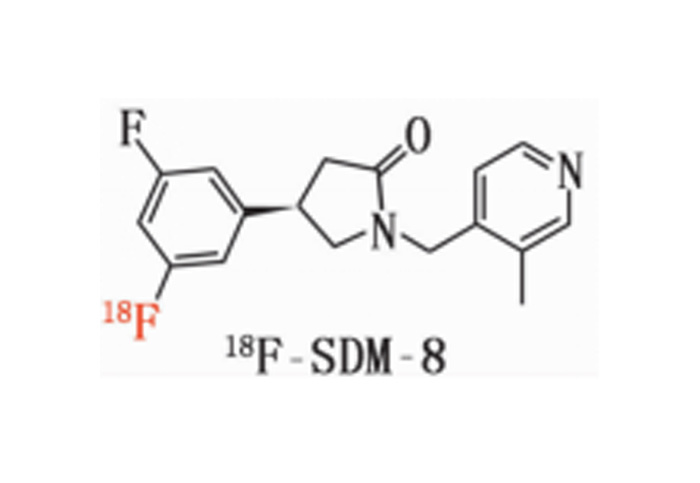
18F-SDM-8 material
Keywords: synthetic equipment disposable consumables
Classification:
Tracers

Hotline:
18F-SDM-8 material
Graphic Details
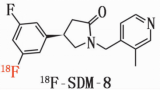
18F- SDM -8 Materials
1. Drug Name (Generic Name, Chemical Name, English Name, Pinyin, and rationale for custom names if any)
Generic Name: 18F- SDM -8
Chemical Name: (R)-4-(3 - fluoro - 5-( fluoro - 18F) phenyl )-1-((3 - methylpyridine - 4 - yl ) methyl ) pyrrolidine - 2 - one
English Name: (R)-4-(3-Fluoro-5-(fluorine-18F) phenyl)-1-((3-methylpyridin-4-yl) methyl) pyrrolidin-2-one
Pinyin: (R)-4-(3-fú - 5-(fú - 18F)běn jī)-1-((3-jiǎ jī bǐ dìng - 4-jī)jiǎ jī)bǐ luò wán - 2-tóng
2. Drug Chemical Structure, Molecular Weight, Molecular Formula
Chemical Structure:
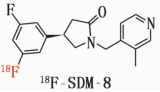
Molecular Weight: 317
Molecular Formula: C 18 H 19 F 2 N 2 O
3. Rationale ( Literature on the research and application of this product at home and abroad )
Research and Application Abroad
- Research: Abroad, 18F-SDM-8 is being researched as a novel positron emission tomography (PET) radiotracer. Related synthesis and in vivo evaluation studies have shown that its in vitro binding test shows high binding affinity for synaptic vesicle glycoprotein 2A (SV2A) (Ki = 0.58 nM), with high molar activity (241.7 MBq/nmol) and high radiochemical purity (>98%) during preparation. In rhesus monkey brain studies, it showed high uptake, with peak standardized uptake values (SUV) greater than 8, and rapid reversible kinetic characteristics. Through displacement and blockade studies with levetiracetam, 11C-UCB-J, etc., the reversibility and specificity of its binding to SV2A were confirmed.
- Application: Mainly focuses on neurodegenerative and mental illnesses. Because it can target SV2A, and synaptic structure damage and changes are related to many brain diseases, such as Alzheimer's disease, epilepsy, depression, and schizophrenia, 18F-SDM-8 is expected to become a biomarker for measuring synaptic density, assisting in the diagnosis and assessment of these diseases. At the same time, in multimodal imaging studies, it is used in combination with other tracers (such as 18F-FDG) to explore the spatiotemporal relationship between different neuropathological events to elucidate the pathological process of neurodegenerative diseases.
Research and Application in China
- Research: There are also relevant research explorations on 18F-SDM-8 in China. Improvements may be made in the synthesis process to meet the quality and quantity requirements of preclinical research and future clinical applications.
- Application: Studies have used 18F-SDM-8 for imaging of a rat temporal lobe epilepsy model. By performing 18F-SDM-8 and 18F-FDG microPET/CT imaging at different time points (acute phase, latent phase, chronic phase) in rats with epilepsy induced by alginate injection, the asymmetry index (AI) was calculated to evaluate its ability to identify epileptic foci. The results showed that there was a statistically significant difference in the AI of the hippocampus between the epilepsy group and the control group in imaging at different time points, and the imaging agent showed asymmetrical uptake in the epilepsy group, consistent with the pathological staining results, showing its application potential in the detection of epileptic foci.
4 Research methods, experimental conditions, etc., of imaging or simulated clinical functional determination tests of target organs and the whole body of experimental animals, and imaging or functional determination results at various time phases
[Drug Name]
Generic Name: 18F-SDM-8
Chemical Name: (4R)-4-(3-(¹⁸F)fluoranyl-5-fluorophenyl)-1-[(3-methylpyridin-4-yl)methyl]pyrrolidin-2-one
English Name: 18F-Sdm-8
Pinyin: shíbā F-S-D-M-bā
[Ingredients]
The main component and its chemical name are: Synaptic Vesicle Glycoprotein 2A (SV2A)
Its structural formula is:
[Properties]
This product is a colorless or slightly yellow, clear liquid.
Indications: Epilepsy focus localization; lesion detection in focal cortical dysplasia (FCD); synaptic density assessment in central neurodegenerative diseases; other neurological disease research.
18F-Sdm-8 PET/CT Brain Imaging Procedure
I. Pre-examination Preparation
Diet and medication management:
Avoid strenuous exercise within 24 hours before the examination, and fast for 4-6 hours (water is allowed) to reduce blood glucose interference.
Diabetic patients need to control their blood glucose in advance (fasting blood glucose is recommended to be ≤11 mmol/L), and adjust the time of hypoglycemic medication if necessary.
Medical history collection and contraindication screening:
Provide previous imaging reports (such as MRI, CT), pathology results, and medication records.
Confirm that there is no claustrophobia, severe pain, or agitation that would prevent cooperation with the examination.
Environmental and clothing requirements:
Change into clothing without metal ornaments before the examination, and remove dentures, hair clips, and other interfering objects.
Keep the environment warm to avoid cold stimulation causing brown fat to uptake the imaging agent.
II. Radiopharmaceutical Injection and Rest Period
Drug injection:
Intravenous injection of 18F-SDM-8 (dosage adjusted according to weight and equipment requirements), remain seated or supine after injection.
Resting wait:
After injection, rest for 45-60 minutes in a dark, quiet environment, avoid talking, chewing, or mental activity to ensure that the imaging agent is evenly distributed in the brain.
Drink plenty of water to promote the metabolism of the imaging agent, and empty the bladder before the examination.
PET/CT acquisition: Scanning should be performed on time 50 minutes after injection of the imaging agent. Static scanning time is 50 minutes after injection, and acquisition is 20 minutes. Dynamic acquisition time is 0-70 minutes. CT and PET acquisition parameters and reconstruction methods should be consistent with brain 18F-FDG imaging. The scanning group needs to strictly control the injection time and scanning time, and strictly record the scanning time.
Image interpretation and analysis: Interpret images promptly.
Report: Issue a report promptly.
Follow-up arrangements: 18F-Sdm-8 and 18F-FDG brain imaging should be spaced at least 10 half-lives (or 20 hours) apart.
This product can only be used in medical institutions with a "Radioactive Pharmaceutical Use License".
Adverse Reactions
None found.
Contraindications
None found.
Precautions
If discoloration or turbidity occurs, discontinue use.
This product can only be used in medical institutions with a "Radioactive Pharmaceutical Use License".
Use in Pregnant and Lactating Women
Contraindicated in pregnant and lactating women.
Pediatric Use
Reduce dosage appropriately according to weight.
Specifications
0.37~7.40GBq.
Storage and Packaging
This product is sealed in a 30ml vial and placed in a lead container.
Shelf Life
6 hours from the calibration time.
Manufacturer
Name: Hangzhou Jirei Technology Co., Ltd.
Address: No. 319, Shenjia Road, Gongshu District, Hangzhou, Fengqi Valley Yunzhang Industrial Park
Postal Code: 234122
Telephone Number: 0571-87701916
Previous Page
18F-JR1004 material
Next Page
Related Products
Consulting