Products
Built multiple sets of modular, multi-functional intelligent drug automatic synthesis platform
OnePlatform M/F
The OnePlatform M/F Automated Synthesizer specializes in the preparation of metallic radionuclide drugs, transforming complex labeling procedures for metals such as 68Ga, 64Cu, and 177Lu into standardized, fully automated processes. It boasts advantages like broad compatibility with various metallic radionuclides, high labeling efficiency, and consistently stable product quality, making it ideal for meeting the R&D and production needs of metallic radionuclide-based drugs used in clinical nuclear medicine diagnostics and therapies.
Keywords: synthetic equipment disposable consumables
Classification:
Product Center
Synthesis Equipment

Hotline:
OnePlatform M/F
Graphic Details
I. Equipment Overview
The OnePlatform M/F Automated Synthesizer specializes in the preparation of metallic radionuclide drugs, transforming complex labeling procedures for metals such as 68Ga, 64Cu, and 177Lu into standardized, automated processes. It boasts advantages like broad compatibility with various metallic radionuclides, high labeling efficiency, and consistently stable product quality, making it ideal for meeting the R&D and production needs of metallic radionuclide-based drugs used in clinical nuclear medicine diagnostics and therapies.
II. Core Application Scope
- Clinical Diagnosis and Treatment: Supports isotope labeling with 68Ga (for tumor and neuroendocrine disease diagnosis), 64Cu (for synergistic PET imaging and radiotherapy), 177Lu (for the treatment of neuroendocrine tumors and prostate cancer), and more—catering to the production of a wide range of mainstream radiopharmaceuticals.
- Drug development: Supporting small-volume, multi-batch metal radionuclide labeling experiments, and optimizing labeling protocols tailored to various ligand structures (such as DOTA, NOTA, and others).
III. Core Specifications and Performance Advantages
(1) Hardware Configuration
- Reactor and Temperature Control: 1 reactor with independent heating capability (up to 220°C) and air cooling; 1 temperature sensing device for real-time monitoring of the reactor temperature.
- Fluid Control: 6 pinch valves for controlling nitrogen, vacuum, and reactor sealing; 25 three-way valves (4 of which are rotatable for orientation), enabling flexible switching of flow paths; and 5 syringes (2.5 ml, 10 ml, 20 ml, 30 ml, with adjustable injection speed) for precise reagent delivery.
- Gases and Vacuum: 1 vacuum pump (providing vacuum from 0 to -50 kPa), 1 nitrogen gas source (delivering 0.6 MPa pressure); equipped with pressure monitoring functionality to continuously track and regulate both nitrogen and vacuum pressures.
- Safety and Detection: 3 radiation detectors monitor the radiation activity in purification columns and reactors; disposable cartridge-based flow path reagents are used to prevent contamination and minimize radiation exposure.
(II) Software Features
- Process and Records: Graphical programming edits the synthesis workflow; automatically records and saves key parameters of the synthesis process; and generates a comprehensive GMP-compliant report upon completion of the synthesis.
- Detection and Optimization: Automated pre-synthesis detection system; supports full replay of the operational process for post-event analysis and training; also enables pre-purification to remove impurities from metallic radionuclides.
- Adaptability and Control: Compatible with a variety of germanium-gallium generators; can be connected wirelessly or via cable, allowing users to monitor operational status on computers and tablets; also supports network-based remote diagnostics.
IV. Major Synthetic Drugs
It can synthesize a variety of tracers based on different isotopes, including:
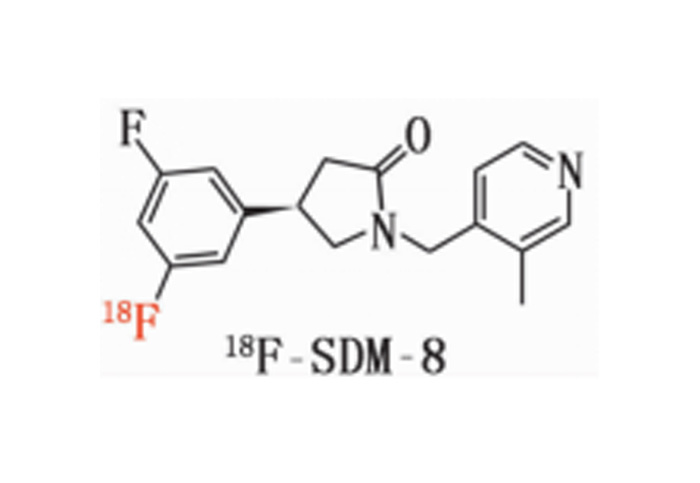
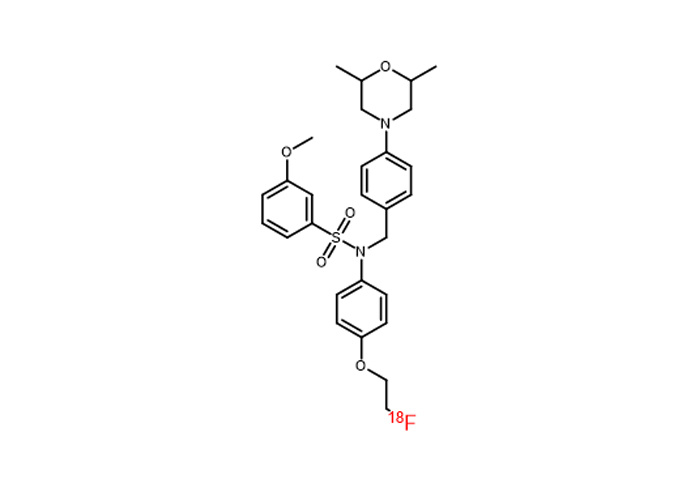
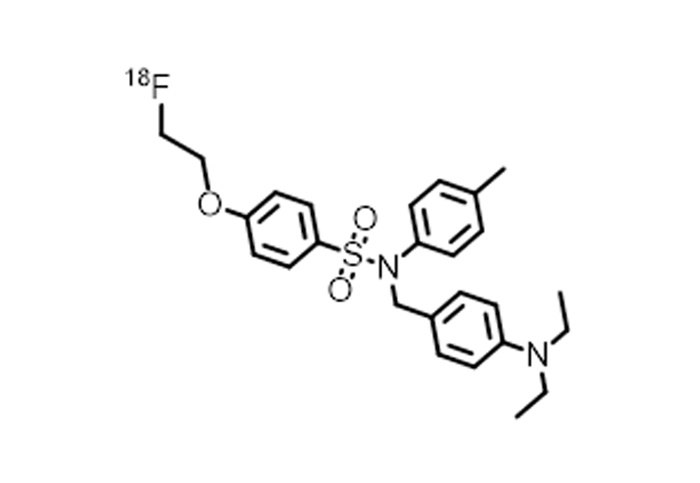
- [¹⁸F]: FDG, F-DOPA, JK-PSMA-7, and more.
- [¹¹C]: Methionine, Choline, PiB.
- [⁶⁸Ga]: DOTA-X, PSMA, Ga-DOTA-TATE, and more.
- [¹⁷⁷Lu]: DOTA - X, PSMA, Lu - OncoFAP - DOTAGA.
- [⁶⁴Cu]: CuCl₂, Cu-DOTA-TATE, DOTA-X.
- [²²⁵Ac], [¹¹¹In]: Ac-PSMA-617, In-PSMA-617, In-RM2.
V. Technical Specification Parameters
|
Specification Category |
Specific parameters |
|
Equipment dimensions (Width × Height × Depth) |
540mm × 420mm × 430mm |
|
Equipment weight |
60kg |
|
Operating Voltage |
220V |
|
Standard Configuration |
Synthetic module integrates chromatography module |
6. Applicable Scenarios
Department of Nuclear Medicine / Clinical Institutions (for on-site preparation of radiopharmaceuticals), Pharmaceutical R&D Companies (focusing on process optimization and pilot-scale production), Research Institutes (dedicated to basic research and ligand screening), and Radiopharmaceutical Manufacturing Enterprises (engaged in large-scale production).
Previous Page
Disposable liquid dispensing box
Next Page
Related Products
Consulting