Products
Built multiple sets of modular, multi-functional intelligent drug automatic synthesis platform
18F-FPABZA material
Keywords: synthetic equipment disposable consumables
Classification:
Tracers

Hotline:
18F-FPABZA material
Graphic Details
18 F-FPABZA Materials
1. Drug Name (Generic Name, Chemical Name, English Name, Pinyin, and rationale for custom names if any)
Generic Name: Fluorine [18 F]- FPABZA
Chemical Name: N- ( 4- (( 2- (Diethylamino)ethyl)carbamoyl) -3- Methoxyphenyl) -5- Fluoropyridine acetamide
English Name: N-(4-((2-(Diethylamino)ethyl)carbamoyl)-3-methoxyphenyl)-5-fluoropicolinamide
Pinyin:
2. Drug chemical structure, molecular weight, molecular formula
Chemical Structure:

Molecular Weight: 388.44
Molecular Formula: C 20 H 26 FN 3 O 3
3. Rationale ( Literature on the research and application of this product at home and abroad )
18F-FPABZA is a novel melanoma-targeted PET probe designed to improve the early detection of melanoma. Melanoma is an aggressive skin cancer, and traditional PET probes (such as 18F-FDG) are difficult to accurately detect melanoma in the early stages due to their non-specificity. Therefore, the development of a PET probe that specifically targets melanin has become a research focus.
18F-FPABZA, as a novel melanoma-targeted PET probe, shows good potential in the early detection of melanoma. However, it has not yet been clinically applied at home and abroad, and further clinical trials are needed to verify its safety and effectiveness.
4. Research methods, experimental conditions, and other data on the target organs and whole-body imaging or simulated clinical functional determination tests of experimental animals, and the imaging or functional determination results at each observation phase
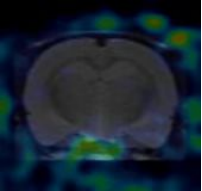
I. Whole-body imaging and delayed imaging of experimental animals
1. Materials and Methods
1.1. Experimental Animals
The experimental animal was one mouse, provided by Hangzhou Medical College, weighing about 200g, male. After injection of the drug, anesthesia was performed before PET scanning, images were collected, and the drug distribution map in the body was obtained. After imaging, the experimental animals gradually woke up and recovered normally, with good diet, defecation, urination, and mental state.
7. Product Instructions
[Product Name]
Generic Name: Fluorine [18F]- FPABZA
Chemical Name: N-(4-((2-(Diethylamino)ethyl)carbamoyl)-3-methoxyphenyl)-5-fluoropyridine acetamide
English Name:
N-(4-((2-(Diethylamino)ethyl)carbamoyl)-3-methoxyphenyl)-5-fluoropicolinamide
Pinyin: Fu[18}-FPABZA
[Ingredients]
Main ingredient: Fluorine-18F, a novel melanin-targeted agent, with the following structural formula:

Colorless to pale yellow clear solution
Indications: Melanoma
[18F-FPABZA PET/C Brain Imaging Procedure]
I. Pre-examination Preparation
Patient Preparation
Fasting for at least 6 hours (water is allowed), avoid strenuous exercise and high-sugar diet to reduce background metabolic interference;
Remove metal head ornaments (such as hair clips, dentures, etc.), and change into examination clothes without metal buttons;
Diabetic patients need to adjust their blood glucose to ≤11 mmol/L in advance;
Preparation and Injection of Imaging Agent
Intravenous injection of 18F-FPABZA (dose calculated according to body weight, usually 5-10 mCi), rest after injection and keep eyes closed to avoid brain activity interfering with imaging;
II. Absorption and Distribution of Imaging Agent
Waiting period: After injection, rest for 45-60 minutes to ensure that 18F-FPABZA fully penetrates the blood-brain barrier and binds to the target (such as β-amyloid protein or other specific molecules);
Urination and drinking water: Empty the bladder before the examination and drink plenty of water to reduce the effect of urinary system radioactivity on the image;
PET/CT acquisition: Scanning should be performed on time 50 minutes after injection of the imaging agent. Static scanning time is 50 minutes after injection, and data acquisition takes 20 minutes. Dynamic acquisition time is 0-60 minutes. CT and PET acquisition parameters and reconstruction methods should be consistent with brain 18F-FDG imaging. The scanning group needs to strictly control the injection time and scanning time, and strictly record the scanning time.
Image interpretation and analysis: Interpret the images in a timely manner.
Report: Issue the report promptly.
Follow-up arrangements: Brain imaging with 18F-FPABZA and 18F-FDG should be spaced at least 10 half-lives (or 20 hours) apart.
This product is only for use in medical institutions with a "Radioactive Pharmaceutical Use License".
[Adverse Reactions]
None have been found.
[Contraindications]
None have been found.
[Precautions]
If discoloration or cloudiness occurs, discontinue use.
This product is only for use in medical institutions with a "Radioactive Pharmaceutical Use License".
[Use in Pregnant and Lactating Women]
Contraindicated in pregnant and lactating women.
[Pediatric Use]
Reduce dosage appropriately according to body weight.
[Specifications]
0.37~7.40 GBq.
[Storage and Packaging]
This product is sealed in a 30ml vial and placed in a lead container.
[Expiration Date]
6 hours from the time of calibration.
[Manufacturer]
Name: Hangzhou Jirei Technology Co., Ltd.
Address: No. 319, Shenjia Road, Gongshu District, Hangzhou, Fengqi Valley Yunzhang Industrial Park
Postal Code: 234122
Telephone Number: 0571-87701916
Previous Page
18F-DPA-714 material
Next Page
Related Products
Consulting