Products
Built multiple sets of modular, multi-functional intelligent drug automatic synthesis platform
18F-AV1451 material
Keywords: synthetic equipment disposable consumables
Classification:
Tracers

Hotline:
18F-AV1451 material
Graphic Details
18F-AV1451 Materials
1. Drug Name (Generic Name, Chemical Name, English Name, Pinyin, and rationale for custom names if any)
Generic Name: Flortaucipir F18
Chemical Name: 7-(6- fluoropyridine -3- yl )-5H- pyridino [4,3-b] indole
English Name: Flortaucipir F18
Fluorine - 18 AV1451
Pinyin: Fú tài pǔ F18
2. Drug Chemical Structure, Molecular Weight, Molecular Formula
Chemical Structure:
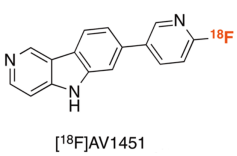
Molecular Weight: 303.3
Molecular Formula: C 16 H 15 18 FN 3 O 2
3. Rationale (Literature on the research and application of this product at home and abroad)
Domestic: Some research teams and hospitals are actively carrying out relevant research and synthesis work. For example, the nuclear medicine department of the First Affiliated Hospital of Harbin Medical University, led by physicist Han Wei, successfully synthesized 18F-AV1451, which has promoted the application of neuro-nuclear medicine molecular imaging in Heilongjiang Province. Director Zhao Yinlong of the Nuclear Medicine Department of the Second Hospital of Jilin University applied 18F-AV1451 imaging to the diagnosis of Alzheimer's disease, providing strong support for the accurate diagnosis of Alzheimer's disease patients.
International: It is the first tau tracer approved by the U.S. Food and Drug Administration. The development process involved extensive experimental research, including animal model and human trials, to verify its specific binding ability to tau protein, safety, and efficacy.
Applications
Domestic: Mainly used in the diagnosis of Alzheimer's disease. For example, Professor Fu Peng's team from the First Affiliated Hospital of Harbin Medical University, in collaboration with the new molecular probe 18F-AV1451, successfully diagnosed Alzheimer's disease. Through PET/MR imaging, it was found that patients had increased 18F-AV1451 uptake in the bilateral parieto-occipital regions, left frontal lobe, bilateral hippocampus, and bilateral temporal lobes, and tau protein imaging was positive, providing important evidence for the diagnosis of the disease.
International: Widely used in Alzheimer's disease-related research and clinical diagnosis. It detects tau protein tangles in the brain through PET imaging, helping doctors with early diagnosis, disease assessment, and research on disease progression. Studies have shown that the classification accuracy of 18F-AV1451 in clinically distinguishing Alzheimer's disease and mild cognitive impairment (MCI) is approximately 85.7%, but there are also problems such as relatively low affinity for non-Alzheimer's tau lesions and off-target effects.
4 Research methods, experimental conditions, and data on whole-body imaging or simulated clinical functional determination tests of target organs in experimental animals, and imaging or functional determination results at various time points of observation
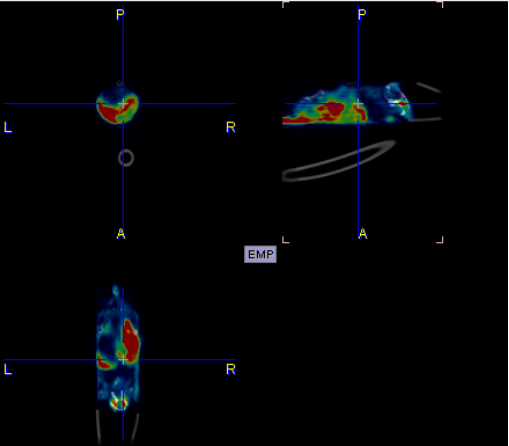
I. Whole-body and delayed imaging of experimental animals
1. Materials and Methods
1.1. Experimental Animals
The experimental animals were mice, weighing approximately 20g, provided by the Experimental Animal Center of Hangzhou Medical College. The scanning location was the PET center of the Second Affiliated Hospital of Zhejiang University. Images were acquired 45 minutes after drug injection to obtain a map of drug distribution in the body. The brain signal was weak, able to penetrate the blood-brain barrier, because in healthy mice, there is almost no tau protein deposition in the brain, making it difficult for the drug to remain.
5. Product Instructions
[Drug Name]
Generic Name:
Fluorine-[18F]-β-Tau Protein Injection
18F-AV1451
Chemical Name: 7-(6-fluoropyridine-3-yl)-5H-pyridino[4,3-b]indole
English Name: Flortaucipir F18
Fluorine - 18 AV1451
Pinyin: Fú tài pǔ F18
[Ingredients]
The main ingredient and its chemical name are: β-Tau protein
Its structural formula is

[Properties]
This product is a yellow liquid.
[Indications]
18F-AV1451 is used for the diagnosis and differential diagnosis of Alzheimer's disease (AD)
[18F-AV1451 PET/CT Brain Imaging Procedure]
Patient Appointment: The clinician must communicate and make an appointment with this center at least one day in advance. The clinician or the appointment doctor of this center should inform the patient that they can eat normally on the day of the examination. If possible, they should fast for 2 hours. AD drugs and related drugs acting on the brain should be discontinued within 24 hours before the AV45 scan. Patients need to arrive at the center one hour before the scheduled appointment time.
Medical History Collection: Brief medical history, clinical diagnosis, informed consent form signed by participants.
Pre-examination Preparation: Instruct the patient to drink water and use the restroom. After injection of the imaging agent and before the examination, they should not drink water or use the restroom again.
Before Injection: The drug group confirms the measurement time and dosage of each injection and reports it to the nursing group. The nursing group confirms the injection time and returns the residue to the drug group. The drug group records the residual dosage and time.
Injection of 18F-AV1451: Only one patient can be injected at a time. The injection dose is 10 mCi (±10%). The nursing group must strictly record the injection time.
Patient Rest: Before the examination, the subject should be instructed to remain quiet, refrain from speaking, and maintain consciousness. Family members or caregivers may accompany the subject during this time, but they should not talk or use electronic devices.
PET/CT Acquisition: Scanning should be performed exactly 50 minutes after injection of the imaging agent. Static scanning time is 20 minutes at 50 minutes post-injection. Dynamic acquisition time is 0-70 minutes. CT and PET acquisition parameters and reconstruction methods should be consistent with brain 18F-FDG imaging. The scanning team must strictly control the injection time and scanning time, and strictly record the scanning time.
Image Interpretation and Analysis: Interpret images promptly.
Report: Issue a report promptly.
Follow-up Arrangements: 18F-AV1451 and 18F-FDG brain imaging should be spaced at least 10 half-lives (or 20 hours) apart.
This product is for use only in medical institutions with a "Radioactive Pharmaceutical Use License".
[Adverse Reactions]
None have been found.
[Contraindications]
None have been found.
[Precautions]
If discoloration or cloudiness occurs, discontinue use.
This product is for use only in medical institutions with a "Radioactive Pharmaceutical Use License".
[Use in Pregnant and Lactating Women]
Contraindicated in pregnant and lactating women.
[Pediatric Use]
Reduce dosage appropriately according to body weight.
[Specifications]
0.37~7.40GBq.
[Storage and Packaging]
This product is sealed in a 30ml vial and placed in a lead container.
[Expiration Date]
6 hours from the time of calibration.
[Manufacturer]
Name: Hangzhou Jirei Technology Co., Ltd.
Address: No. 319, Shenjia Road, Gongshu District, Hangzhou, Fengqi Valley Yunzhang Industrial Park
Postal Code: 234122
Telephone Number: 0571-87701916
Previous Page
18F-AV133 material
Next Page
Related Products
Consulting