Products
Built multiple sets of modular, multi-functional intelligent drug automatic synthesis platform
18F-AV133 material
Keywords: synthetic equipment disposable consumables
Classification:
Tracers

Hotline:
18F-AV133 material
Graphic Details
18F-AV133 Materials
1. Drug Name (Generic Name, Chemical Name, English Name, Pinyin, and rationale for custom names if any)
Generic Name: 18F-AV133
Chemical Name: N-[2-(3,4 - difluorophenyl )-6 - methylpyridine - 3 - yl ]-2-(18F) fluoroacetamide
English Name: 18F-AV133 (Full English name is: N-[2-(3,4-difluorophenyl)-6-methylpyridin-3-yl]-2-(18F)fluoroacetamide )
Pinyin: yī bā F-AV yī sān sān
2. Drug Chemical Structure, Molecular Weight, Molecular Formula
Chemical Structure:
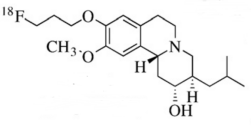
Molecular Weight: 251.23
Molecular Formula: C 12 H 11 F 2 N 3 O
Rationale
Parkinson's disease (PD) is a common neurodegenerative disease. Its main pathological feature is the degeneration of dopaminergic neurons in the substantia nigra-striatum, leading to a decrease in dopamine levels in the brain. The occurrence and development of the disease are closely related to the degree of deficiency of dopamine transporter (DAT) and vesicular monoamine transporter type II (VMAT2). 18F-AV133 is a novel positron emission tomography (PET) imaging agent that specifically binds to VMAT2 and can dynamically display changes in VMAT2 levels in the brain in real time. This helps in the early diagnosis of Parkinson's disease before the onset of clinical symptoms. Early diagnosis is of great significance for early intervention and treatment of Parkinson's disease, allowing patients to gain more treatment time, delaying disease progression, and improving quality of life.
Domestic Research Status
Researchers at the Key Laboratory of Radiopharmaceuticals of the Ministry of Education, Beijing Normal University, have conducted in-depth research in this field1. Zhu Lin et al. synthesized and evaluated 2-amino-dihydrotetrabenazine derivatives as VMAT2 imaging probes in 20091. In 2010, they improved the radiosynthesis method of 18F-AV-1331. In 2012, they further studied the imaging of VMAT2 binding sites in the brain and the influence of false carriers of 18F-AV-1331.
The project "Research on the mechanism of functional and structural neural network evolution in the chronic Parkinson's disease model induced by MPTP in rhesus monkeys" undertaken by Shang Huifang's team at Sichuan University independently synthesized the 18F-AV133 tracer for detecting VMAT2. Through experiments, they found that the uptake rate of 18F-AV133 in various functional regions of the striatum in rhesus monkeys reached a stable state about 60 minutes after the injection of the tracer, and determined that the optimal time window for detecting the uptake value of 18F-AV133 in the striatum of rhesus monkeys was 60 to 125 minutes after injection.
Domestic Application Status
Domestic studies have applied 18F-AV133 to the diagnostic research of Parkinson's disease. Nuclear medicine PET/CT (MR), as an imaging method combining function and anatomy, combined with the 18F-AV133 molecular probe, can detect Parkinson's disease as early as 17 years in advance, providing strong technical support for the early diagnosis of Parkinson's disease.
International Application Status
There are also relevant studies on the application of 18F-AV133 abroad. For example, PET imaging studies using 18F-AV133 have investigated changes in VMAT2 in the brains of Parkinson's disease patients, providing important imaging evidence for the study of the pathophysiological mechanisms of Parkinson's disease. In addition, in the study of other neurological diseases, there have been attempts to use 18F-AV133 to observe changes in VMAT2 in relevant brain regions to explore the pathogenesis of the disease. However, specific application research on 18F-AV133 abroad may vary due to differences in research institutions and projects, generally focusing on its value in the diagnosis and pathological research of neurological diseases.
4 Research methods, experimental conditions, and other data on whole-body imaging or simulated clinical functional determination tests of target organs in experimental animals, and imaging or functional determination results at various time phases of observation
I. Whole-body and delayed imaging of experimental animals
1. Materials and Methods
1.1 Experimental Animals
The experimental animal was one mouse, provided by Huajing, weighing about 23g, male. After injection of the drug, anesthesia was performed for PET scanning, images were collected, and the drug distribution map in vivo was obtained. After imaging, the experimental animals gradually woke up and recovered normally, with good diet, defecation, and mental state.
The following figure shows the 30min imaging image
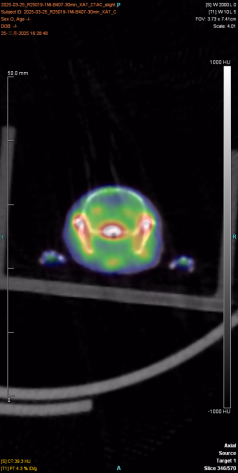 |
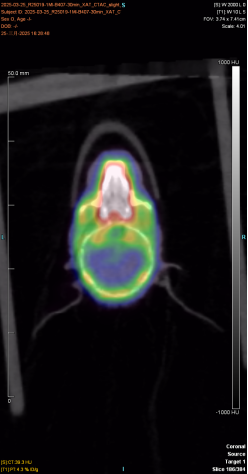 |
4. Drug Instructions
[Drug Name]
Generic Name:
Fluorine [¹⁸F] Promazine
Chemical Name: 18F-9- Fluoropropyl dihydroxybenzothiazole
English Name:
9-[18F]fluoropropyl-dihydrotetrabenazine
Pinyin:
Fú [¹⁸F] Bǐng Nà Qín
【Composition Part }
The main component and its chemical name are: Vesicular monoamine transporter, its structural formula is:
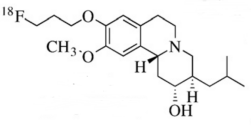
【Appearance】
This product is a colorless and clear solution.
【Indications】
18F- AV 133 Used for Parkinson's disease ( PD ) precise diagnosis and assessment , Lewy body dementia ( DLB ) and Alzheimer's disease ( AD ) differential diagnosis
【 18F-AV133 PET/CT Brain Imaging Process }
I. Appointment and medical history assessment
Make an appointment in advance for the examination. The doctor needs to understand the patient's medical history (such as blood glucose level, allergy history, recent medication).
Diabetic patients need to control their fasting blood glucose (recommended ≤ 11.1 mmol/L )
Diet and medication management
Fasting 4-6 hours (water is allowed), avoid sugary drinks and strenuous exercise
Some patients need to stop using drugs that may interfere with the uptake of imaging agents (such as dopaminergic drugs)
Item preparation
Change into metal-free clothing and remove personal accessories (mobile phones, keys, etc.)
II. Injection of imaging agent and resting period
Intravenous injection of imaging agent
Intravenous injection 18F-AV133 (dose adjusted according to weight), avoid strenuous activity after injection
Resting wait ( 60-90 minutes)
Patients need to rest in a quiet, dark environment to promote the distribution of the imaging agent in the brain target (such as VMAT2 )
Avoid talking or thinking activities to reduce interference from extra brain metabolism
P ET/CT Acquisition: After injecting the imaging agent 50min Scan on time. The static scan time is after injection 50 min ,Acquisition 20 minutes. The dynamic acquisition time is 0-70 minutes. CT and PET Acquisition parameters and reconstruction methods should be consistent with brain 18F- FDG imaging. The scanning group needs to strictly control the injection time and scanning time, and strictly record the scanning time.
Image interpretation and analysis: Interpret images promptly.
Report: Issue a report promptly.
Follow-up arrangements: 18F-AV133 and 18F-FDG Brain imaging should be spaced at least 10 half-lives (or 20 hours).
This product can only be used in medical institutions with a "Radioactive Pharmaceutical Use License".
【Adverse Reactions】
None found yet.
【Contraindications】
None found yet.
【Precautions】
If discoloration or turbidity occurs, stop using this product.
This product can only be used in medical institutions with a "Radioactive Pharmaceutical Use License".
【Use in Pregnant and Lactating Women】
Contraindicated in pregnant and lactating women.
【Pediatric Use】
Reduce the dose appropriately according to body weight.
【Specifications】
0.37~7.40GBq 。
【Storage and Packaging】
This product is sealed in 30ml a vial and placed in a lead container.
【Shelf Life】
Calculated from the calibration time as 6 hours.
【Manufacturer】
Name: Name: Hangzhou Jirui Technology Co., Ltd.
Address: Address: Shenjialu, Gongshu District, Hangzhou City 319 Fengqigu Yunzhang Industrial Park
Postal Code: 234122
Telephone Number: 0571-87701916
Previous Page
18F-AV45 material
Next Page
Related Products
Consulting