Products
Built multiple sets of modular, multi-functional intelligent drug automatic synthesis platform
18F-JR1002 material
Keywords: synthetic equipment disposable consumables
Classification:
Tracers

Hotline:
18F-JR1002 material
Graphic Details
18F-JR1002 material
1. Drug name (generic name, chemical name, English name, Pinyin, if there is a customized name, the basis of naming should be explained)
common name:
18F-JR1002
chemical name:
N-(4-(diethylamino)benzyl)-4-(2-(fluoro-18F)ethoxy)-N-(p-tolyl)benzenesulfonamide
pin yin:
18F-JR1002
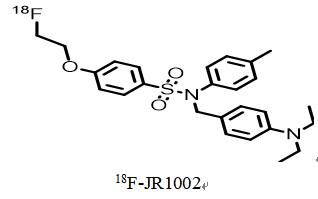
- Chemical structure, molecular weight and molecular formula of the drug
chemical constitution:
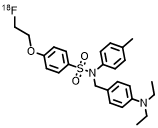
18F-JR1002
3. Basis of the topic (literature on the development and application of this product at home and abroad)
Cannabinoid receptor type 2 (CB2R) belongs to the G protein-coupled receptor (GPCR) family. Together with cannabinoid receptor type 1 (CB1R), they form the endocannabinoid system (ECS) through their respective signaling pathways, constituting a complex and multifunctional endogenous signaling system. Since the cloning of CB2R in 1993, CBRs have been primarily distinguished based on their biological distribution: CB1R is mainly highly expressed in the nervous system and has neuroregulatory properties, while CB2R is widely distributed in the peripheral immune system and has immunomodulatory properties.
Positron emission tomography (PET) is a non-invasive and highly sensitive molecular imaging technique that allows for in vivo quantification of biochemical and pharmacological processes under both healthy and diseased conditions. By using radiolabeled PET tracers, in vivo imaging at the molecular level can be achieved to obtain quantitative information on target engagement and receptor occupancy at specific drug doses. PET imaging combined with specific probes provides real-time information on tracer accumulation in the region of interest, playing a crucial role in precision medicine and quantitative pharmacokinetic studies. Therefore, targeted PET molecular imaging of CB2R receptors holds promise for personalized diagnosis and therapeutic monitoring in various diseases such as neuroinflammation, neurodegeneration, and cancer.
4. Research methods, experimental conditions and other data of target organs and whole body imaging or simulated clinical function measurement tests of experimental animals, and imaging or functional measurement results observed at various phases of the test
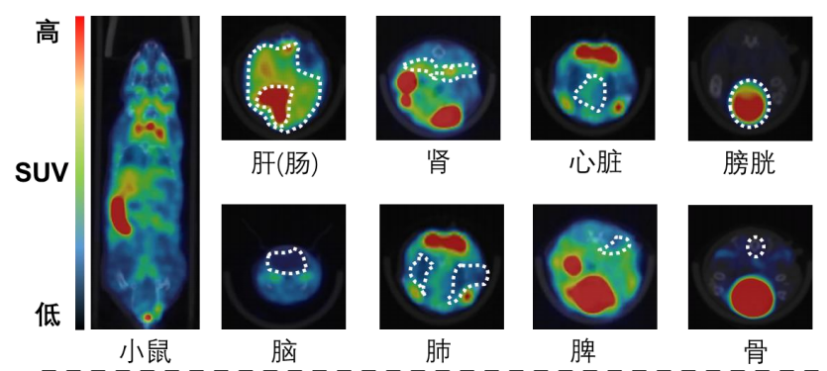
I. Whole body imaging and delayed imaging of experimental animals
1. Materials and methods
1.1 Experimental animals
The experimental animals were two mice with a weight of about 25g and male, obtained from the laboratory of Zhejiang University. Images were collected at multiple time periods after drug injection to obtain the distribution map of the drug in vivo. After imaging, the experimental animals gradually woke up and returned to normal, with good diet, two excreta and mental state.
The figure below shows the 30min imaging image
2. Acute toxicity test
Take 10 healthy mice aged 6-8 weeks with a body weight of 20-25g, half male and half female, and inject 0.5mL of 18F-JR1002 with an activity of 1mCi via the tail vein. For the control group, take 10 healthy mice aged 6-8 weeks with a body weight of 20-25g, half male and half female, and inject 0.5mL of normal saline via the tail vein. Observe the survival status of both groups of mice; there was no statistically significant difference in the survival status within 7 days.
5. Instructions for drugs
Generic name: 18F-JR1002
Chemical name: N-(4-(diethylamino)benzyl)-4-(2-(fluoro-18F)ethoxy)-N-(p-tolyl)benzenesulfonamide
Pinyin: shíbā F-JR yī líng líng èr
[element]
The main component and its chemical name of this product are: G protein and its structural formula
[shape and properties]
This product is a colorless clear or slightly yellow clear solution.
[indication]
18F-JR1002 is used for tumor diagnosis and staging evaluation, cardiovascular disease imaging, bone lesion or bone metastasis monitoring, etc
[18F-JR1002 PET/CT brain imaging process]
Preparation before the examination
Fasting requirements: The examinee should fast for 4-6 hours (water is allowed) to avoid blood glucose fluctuations that interfere with the distribution of the imaging agent.
Blood glucose monitoring: blood glucose should be tested before injection, usually requiring control of less than 11mmol/L.
Disinfection adjustment: suspend drugs that may interfere with the results (such as insulin, hormone drugs), and avoid strenuous exercise.
Imaging agent injection and resting period
Intravenous injection: 18F-JR1002 tracer was injected intravenously, and the dose was adjusted according to body weight and purpose of examination.
Resting waiting: Rest in a quiet, dark environment for 45-60 minutes after injection, during which avoid activity or mental stimulation to ensure the specific distribution of the tracer in brain tissue.
Brain imaging scan
Position fixation: the patient lies on the scanning bed, and the head is fixed with a special head support to keep breathing stable and body still.
CT scan: A low-dose CT scan (about 10 minutes) is performed first to obtain images of the brain's anatomical structure.
PET scan: A PET scan (about 20-30 minutes) was performed to detect the metabolic distribution and targeted binding of 18F-JR1002.
Image processing and diagnosis
Data fusion: PET functional images and CT anatomical images are fused through software to generate 3D brain metabolic imaging.
Clinical interpretation: The nuclear medicine physician issues a diagnosis report based on metabolic activity, lesion location and patient history.
Precautions after inspection
Radiation protection: it is recommended to drink more water and urinate more after examination to accelerate the excretion of radioactive tracer.
Results obtained: The official report is usually received within 1-2 working days.
Follow-up: 18F-JR1002 and 18F-FDG brain imaging should be spaced at least 10 half-lives (or 20 hours).
This product is only for use in medical units with a radioactive Drug Use License.
[untoward effect]
Not yet found.
[taboo]
Not yet found.
[matters need attention]
If the product changes color or becomes cloudy, stop using it.
This product is only for use in medical units with a radioactive Drug Use License.
[Pregnant and lactating women]
Pregnant and lactating women are prohibited from using.
[Medication for children]
Reduce the dose appropriately according to body weight.
[specifications]
0.37~7.40GBq。
[Storage and packaging]
This product is sealed in a 30ml vial and placed in a lead container.
[term of validity]
The time from calibration is calculated as 6 hours.
[production unit]
Name: Hangzhou Jirui Technology Co., LTD
Address: Fengqigu Yunzhang Industrial Park, No.319 Shenjia Road, Gongshu District, Hangzhou City
Zip code: 234122
Phone number: 0571-87701916
Previous Page
18F-FPABZA material
Next Page
Related Products
Consulting