Products
Built multiple sets of modular, multi-functional intelligent drug automatic synthesis platform
OnePlatform Version 3.2
Keywords: synthetic equipment disposable consumables
Classification:
Synthesis Equipment

Hotline:
OnePlatform Version 3.2
Graphic Details
I. Equipment Overview
OnePlatform 3.2 Radiochemical Edition Multifunctional The automated synthesizer is an advanced, automation-based device specifically designed for the field of nuclear medicine, with its core functionality centered on the preparation of radiopharmaceuticals and PET Tracer synthesis. Developed through a standardized system, this equipment transforms complex radioactive chemical labeling procedures into a simple, reproducible, and reliable production process, featuring Easy to operate, wide range of applications, and high production efficiency Three core advantages that can meet the diverse preparation needs of various radionuclide drugs and tracers in both clinical and research settings.
II. Core Application Scope
- Preparation of Complex Radionuclide Pharmaceuticals : Primarily supports
- Metal Isotope Labeling : Achievable
- PET Tracer Synthesis : Compatible with Current Mainstream
III. Core Configuration and Performance Advantages
(1) High-Efficiency Reactor
- Heating system
- Heat-conduction method: Employs a metal-based thermal design for highly efficient heat transfer;
- Power parameters: Power exceeds 100 watts, enabling rapid heating to the target temperature;
- Temperature Limit: Up to
- Cooling System
- Cooling method: Water-cooled cooling, ensuring a gentle operation with low noise levels;
- Safety Advantage: Effectively reduces the risk of radiation leakage, enhancing the safety of the operational environment.
(II) Flexible Process Configuration
- Valve assembly configuration: Integrated 20 A three-way valve that allows flexible switching of the reaction flow path;
- Injection system: Equipped with 5 A syringe designed to meet the precise dispensing needs of various reagents;
- Adaptability: Compatible with various types
(III) Integrated Chromatography Module
- Core Components
- Detection System: Built-in radiation detector (for real-time monitoring of radioactive material separation), with optional multi-wavelength UV detector;
- Elution System: Features a dual-pump design that supports gradient mixing of elution solutions; equipped with a switching valve, allowing seamless transitions between different mobile phases for efficient elution.
- Functional Advantages : Achieve efficient separation, purification, and detection of the target product, ensuring the purity and consistent quality of the synthesized compound.
(4) Disposable Reagent Kit Card Holders
- Compliance : Press
- Security
- Avoid cross-contamination: Disposable consumables designed to eliminate the risk of contamination caused by reuse;
- Reduce radiation exposure: Minimize direct contact between operators and radioactive materials to lower the risk of radiation exposure.
- Convenience : Simply install the kit onto the synthesizer and add the reagents as instructed to start production—streamlining the process and boosting efficiency.
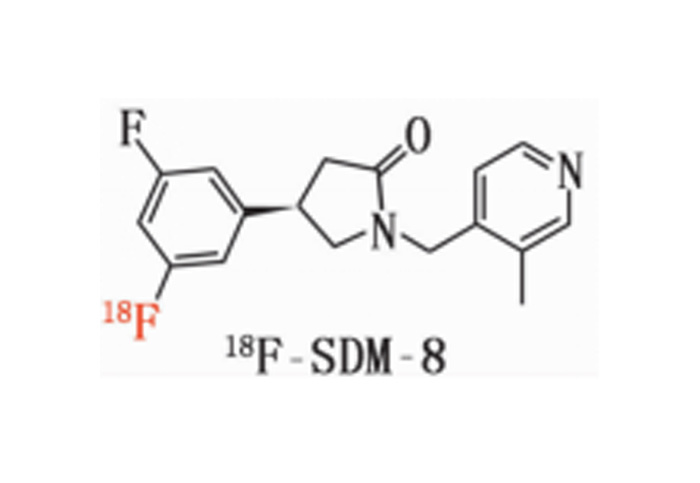
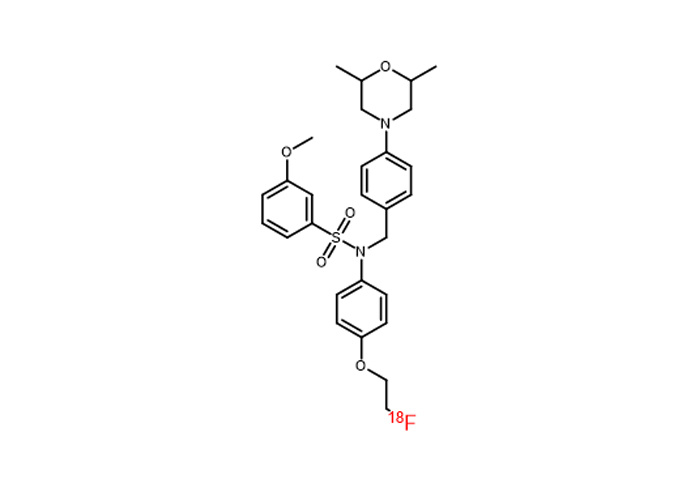
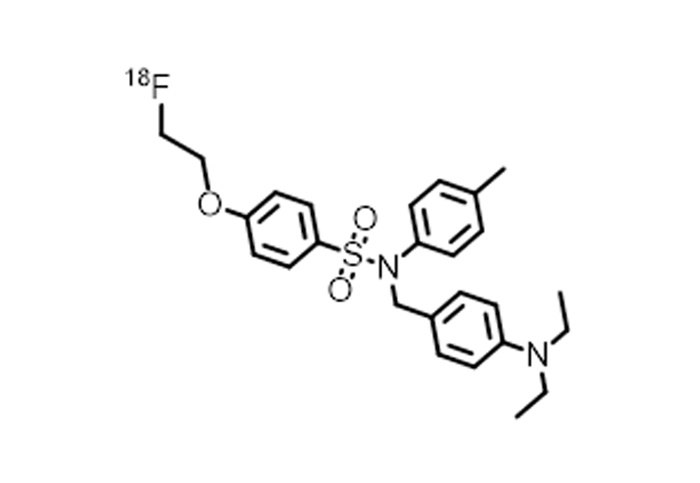
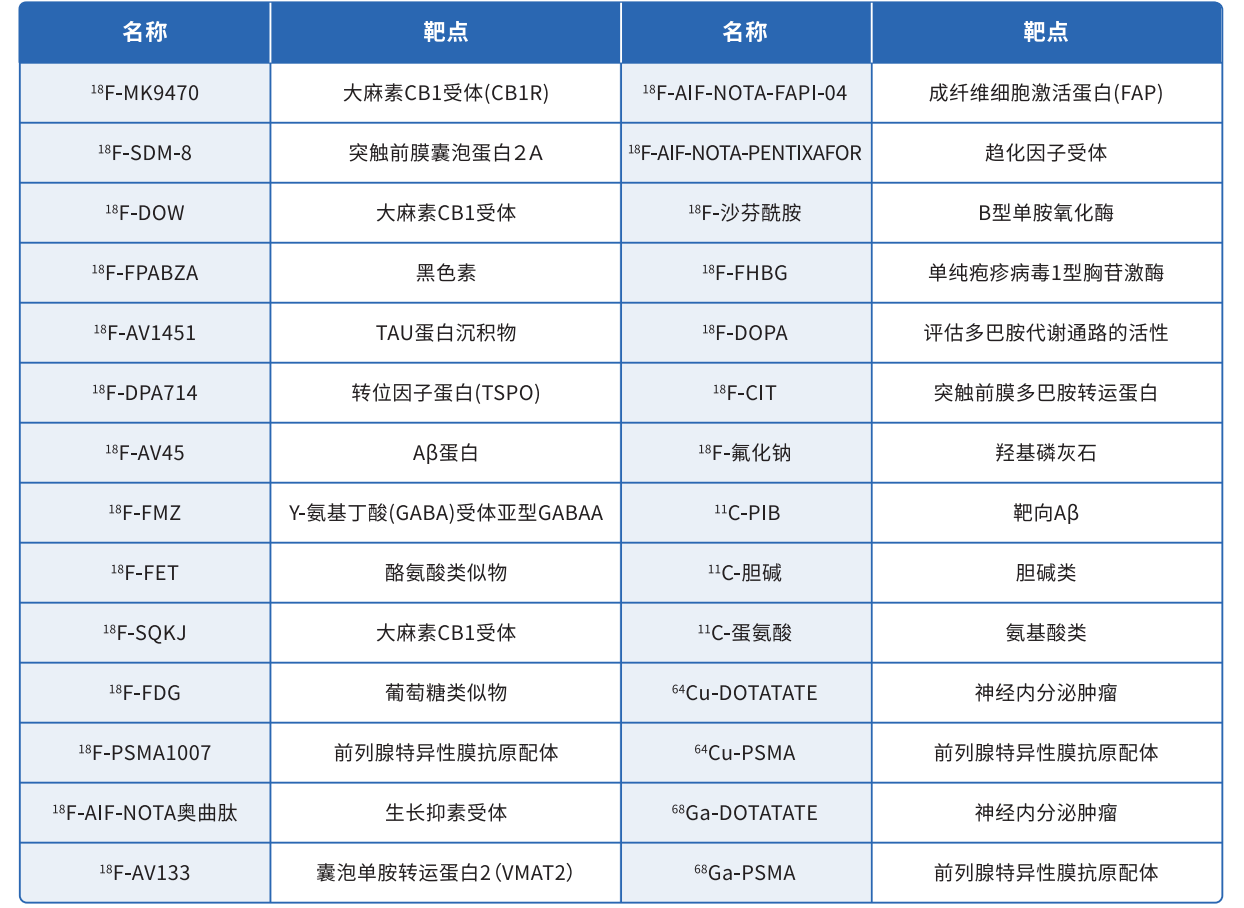
(5) Adaptation 18 F Tracer Type
Has achieved implementation of the following mainstream 18 F The synthesis and adaptation of tracers allow for direct integration into relevant production processes.
IV. Technical Specification Parameters
| Specification Category |
Specific parameters |
| Equipment dimensions (width × High × Deep) |
510mm × 510mm × 500mm |
| Equipment weight |
45kg |
| Operating Voltage |
220V |
| Standard Configuration |
Synthetic module + Chromatography Module |
5. Applicable Scenarios
- Nuclear Medicine Clinical Facility: Used for
- Pharmaceutical R&D companies: Small-scale and pilot-scale production processes in radiopharmaceutical drug development;
- Research institutions: Scientific research experiments and technology development in fields related to nuclear medicine and radiochemistry.
Previous Page
None
Next Page
Related Products
Consulting