Products
Built multiple sets of modular, multi-functional intelligent drug automatic synthesis platform
18F-AV45 material
Keywords: synthetic equipment disposable consumables
Classification:
Tracers

Hotline:
18F-AV45 material
Graphic Details
18 F-AV45 Material
1. Drug Name (Generic Name, Chemical Name, English Name, Pinyin, and Basis for Naming if a Custom Name Exists)
Generic Name: Fluorine [18 F]- β -Amyloid protein Injection
Chemical Name: ( (E)-4-(2-(6-(2-(2-(2-18F- fluoroethoxy ) ethoxy ) ethoxy ) pyridine - 3- yl ) vinyl )-N- methylbenzenamine (( E ) - 4- ( 2- ( 6- ( 2- ( 2- ( 2-18F- f fluoroethoxy ) e thoxy ) e thoxy ) pyridine-3-yl ) vinyl ) -N-methylbenzenamine )
English Name: Fluorine18(18F)-β- am yloidprotein,Aβ
Pinyin: Fu[ 18 F] β-dingfenyangdanbai Zhusheye
2. Chemical Structure, Molecular Weight, and Molecular Formula of the Drug
Chemical Structure:
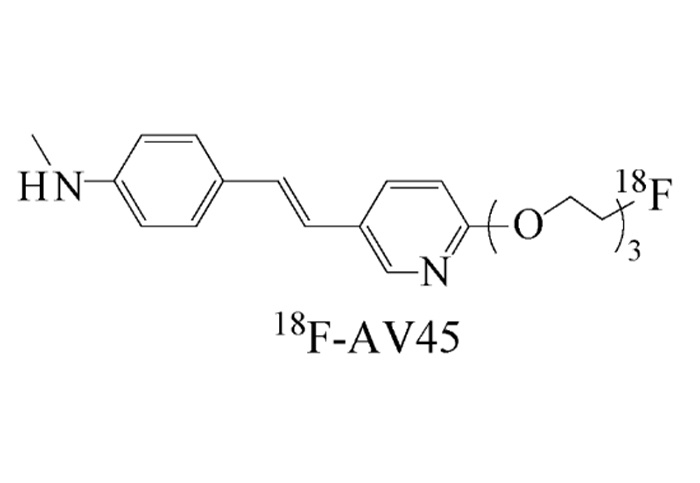
Molecular Weight: 359.19
Molecular Formula: C 20 H 25 18 FN 2 O 3
3. Research Basis (Literature on the Research and Application of This Product at Home and Abroad)
Domestic
Research: Domestic research teams have conducted relevant research in the field of radiopharmaceuticals. For example, Academician Liu Boli's team at Beijing Normal University has developed a new brain plaque imaging agent that is expected to have independent intellectual property rights in China. They found that the comprehensive properties of the 18F-labeled benzoxazole derivative (18F)ZB-35a in vivo and in vitro are comparable to those of 18F-AV45, and preclinical research was underway at that time.
Application: Many hospitals have used 18F-AV45 for clinical diagnosis. For example, the PET center of Huashan Hospital affiliated with Fudan University has launched a distinctive business in the early diagnosis and differential diagnosis of dementia, including 18F-AV-45, in its East Campus. The Nuclear Medicine Department (PET Center) of Xiangya Hospital of Central South University also uses 18F-AV45 for Alzheimer's disease diagnosis and has conducted the first 18F-SDM8 nerve synapse imaging in China, reaching an internationally leading level. In addition, the Han Wei physicist team of the Nuclear Medicine Department of Harbin Medical University No. 1 Hospital successfully synthesized the 18F-AV45 amyloid protein imaging agent. The use of this imaging agent and other high-tech medical technologies provides clinical doctors with accurate examination results and helps patients develop better treatment plans.
Foreign
Research: Early studies used in vitro binding assays, autoradiography, etc., to investigate the binding of 18F-AV-45 in post-mortem AD brain tissue, as well as its biodistribution in mouse and monkey brains. At that time, the research showed that 18F-AV-45 has high binding affinity and specificity for Aβ plaques, and was considered a useful PET agent for detecting Aβ plaques in the living human brain during Phase III clinical trials.
Application: 18F-AV45 is mainly used in foreign countries in the diagnosis and research of Alzheimer's disease, etc., using PET imaging to detect β-amyloid protein deposition in the brain, helping doctors to make early diagnoses, assess the condition, and conduct research on Alzheimer's disease.
4. Drug Instructions
[Drug Name]
Generic Name:
Fluorine [18F]-β-amyloid protein injection
Chemical Name:
(E)-4-(2-(6-(2-(2-(2-18F-fluoroethoxy)ethoxy)ethoxy)pyridine-3-yl)vinyl)-N-methylbenzenamine
English Name:
Fluorine18(18F)-β-amyloidprotein,Aβ
Pinyin:
Fu[18F] β-dingfenyangdanbai Zhusheye
[Ingredients]
The main components and chemical names of this product are: β -Amyloid protein The structural formula is:
The structural formula is:

[Properties]
This product is a colorless and clear or slightly yellow and clear solution.
[Indications]
18F-AV45 For brain diseases: Amyloid protein imaging, used for A D etc.
【 18F-AV45 PET/CT Brain Imaging Process ]
Patient Appointment: The clinician must communicate and make an appointment with the center's doctor at least one day in advance. The clinician or the center's appointment doctor should inform the patient that they can eat normally on the day of the examination. If possible, they should fast. 2h。 A V 45 Before Scan 24h Discontinued A D and related drugs acting on the brain. Patients need to arrive at the center in advance of the scheduled appointment time 1 hours.
Medical History Collection: Brief medical history, clinical diagnosis, informed consent signed by participants.
Pre-examination preparation: Inform the patient to drink water and use the restroom. After the injection of the imaging agent and before the examination, they should not drink water or use the restroom again.
Before Injection: The medication group confirms the measurement time and dosage of each injection and reports it to the nursing group. The nursing group confirms the injection time and returns the residue to the medication group. The medication group records the residual dosage and time.
Injection 18F- A V45 : Only one patient can be injected at a time. The injection dose is 10 mCi (± 10% ). The nursing group must strictly record the injection time.
Patient Rest: Before the examination, the subject should be asked to remain quiet, not speak, and maintain consciousness. During this time, family members or nursing staff may accompany, but please do not talk or use electronic products.
P ET/CT Collection: Needs to be scanned on time after injecting the imaging agent 50min minutes after injection. 50 min ,Collection 20 minutes. Dynamic acquisition time is 0-70 minutes. CT and PET Acquisition parameters and reconstruction methods should be consistent with brain 18F- FDG imaging. The scanning group must strictly control the injection time and scanning time and strictly record the scanning time.
Image Interpretation and Analysis: Interpret images promptly.
Report: Issue a report promptly.
Follow-up arrangements: 18F-AV45 and 18F-FDG Brain imaging should be spaced at least 10 half-lives (or 20 hours).
This product can only be used in medical institutions with a "Radioactive Drug Use License".
[Adverse Reactions]
None have been found.
[Contraindications]
None have been found.
[Precautions]
If discoloration or turbidity occurs, stop using this product.
This product can only be used in medical institutions with a "Radioactive Drug Use License".
[Use in Pregnant and Lactating Women]
Contraindicated in pregnant and lactating women.
[Pediatric Use]
Reduce the dose appropriately according to body weight.
[Specifications]
0.37~7.40GBq 。
[Storage and Packaging]
This product is sealed in a 30ml vial and placed in a lead container.
[Expiration Date]
Calculated from the calibration time as 6 hours.
[Manufacturer]
Name: Name: Hangzhou Jirei Technology Co., Ltd.
Address: Address: Shenjia Road, Gongshu District, Hangzhou City 319 Fengqigu Yunzhang Industrial Park
Postal Code: 234122
Telephone Number: 0571-87701916
Previous Page
18F-SDM-8 material
Next Page
Related Products
Consulting